NMR Spin
MRI Knowledge Hub
What is NMR Spin
Spin is defined as angular moment associated with subatomic particles. All the protons, comprising any atomic nucleus, have the intrinsic quantum property of spin, an intrinsic angular momentum analogous to the classical angular momentum of a top (A conical child’s plaything tapering to a steel point on which it can be made to spin). or spinning sphere. The overall spin of the nucleus is determined by the quantum number S. If the numbers of both the protons and neutrons in a given nuclide are then S = 0 or no spin. For nuclei of even mass number, the multiple is an integer; for those of odd mass number, the multiple is a half-integer. In quantum mechanics spin is now recognized as an intrinsic property of all subatomic particles. Spin is one of the main criteria used to classify particles into two main groups: fermions, with half-integer values of spin (1/2, 3/2, …), and bosons, with integer values of spin (0, 1, 2,…). In the Standard Model all of the “matter” particles (quarks and leptons) are fermions, whereas “force” particles such as photons are bosons. These two classes of particles have different symmetry properties that affect their behaviour.
Spin Angular Momentum
1H, the most commonly used spin-1/2 nucleus in NMR investigation, has been studied using many forms of NMR. Hydrogen is highly abundant, especially in biological systems. It is the nucleus most sensitive to NMR signal (apart from which is not commonly used due to its instability and radioactivity). Proton NMR produces narrow chemical shift with sharp signals. Fast acquisition of quantitative results (peak integrals in stoichiometric ratio) is possible due to short relaxation time. The 1H signal has been the sole diagnostic nucleus used for clinical magnetic resonance imaging (MRI).
Spin Top

Spin

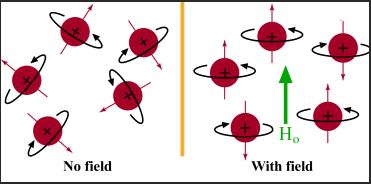
Spin of protons with and without Magnetic Field
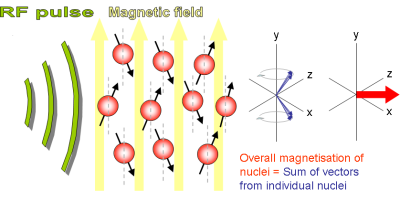
Applying RF field to Spinning protons