NMR/MRI Principle
MRI Knowledge Hub
Journey to Nuclear Magnetic Resonance (NMR)
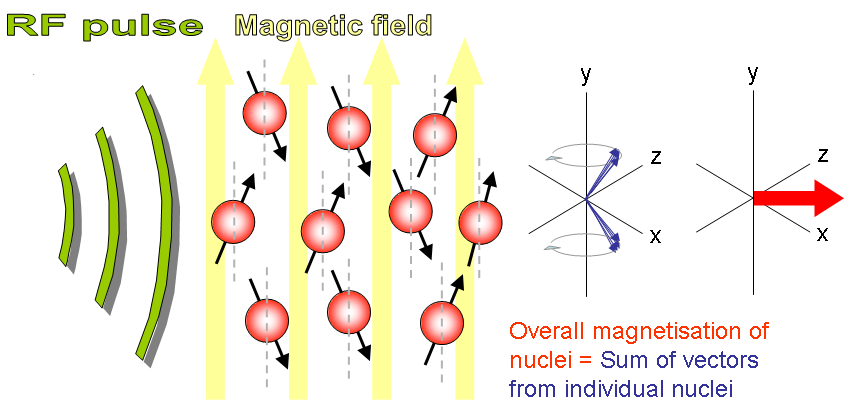
- NMR principle States that when a selected proton sample (like 1H, 13C, 31P etc) placed in strong magnetic field, and if this sample exited with RF energy at larmor frequency, it releases RF enegy back in the form of echo signal which can also be seen as NMR spectra.
- This work was carried by Felix Bloch and Edward Purecell in the year 1945 and awarded Nobel prize in Physics for this work in 1952.
Zeeman in 1950 observed the behaviour of certain nuclei when subjected to a strong magnetic field, called “Zeeman effect”. was only made in the 1950s when NMR spectrometers became commercially available.
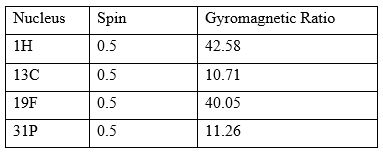
It is a research technique that exploits the magnetic properties of certain atomic nuclei (Refer Table of nuclei, Spin, Gyromagnetic Ratio). The NMR spectroscopy determines the physical and chemical properties of atoms or molecules.
NMR Spectroscopy Working
- First Place the sample in a magnetic field.
- Excite sample into nuclear magnetic resonance with the help of radio frequency to generate NMR signals.
- Detect NMR signals with radio receivers.
- The resonance frequency of an atom in a molecule is changed by the intramolecular magnetic field surrounding it.
- Determine the details of a molecule’s individual functional groups and its electronic structure.
- Nuclear magnetic resonance spectroscopy identify monomolecular organic compounds.
- Spectroscopy method provides details of the structure, chemical environment and dynamics of a molecule.
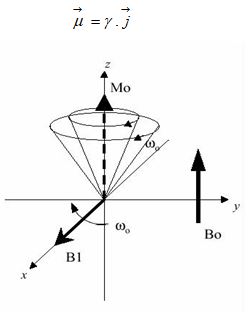
Relationship between Gyromagnetic Ratio, Larmor Frequency, Magnetic Field
Gyromagnetic Ratio
There is a constant of proportionality, known as the gyromagnetic ratio, is dependent on the nucleus type. The values for select nuclei are listed in Table. Most clinical applications of MRI require an abundance of hydrogen inside the object to be imaged because its relatively high gyromagnetic ratio eases the imaging process. The human body is primarily fat and water, both of which have many hydrogen atoms.
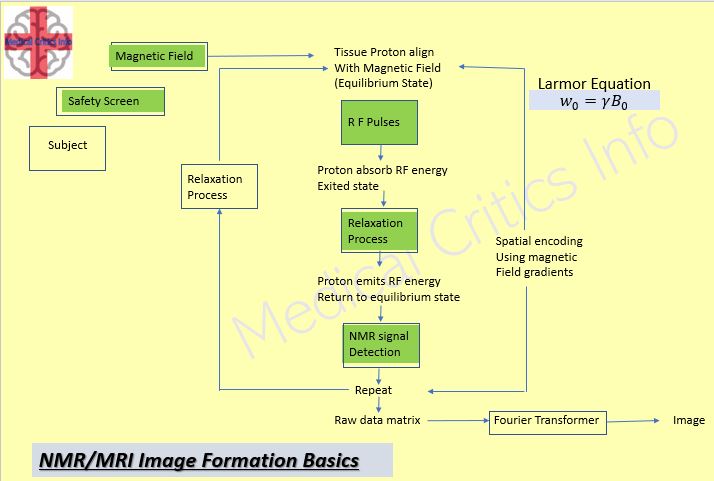
Magnetic Resonance Images formed based on NMR principle
Interaction of High fixed Magnetic, RF field, and Gradient Fields and Sample are essential required to form an MR Image.
What is NMR
NMR is an abbreviation for Nuclear Magnetic Resonance. An NMR instrument allows the molecular structure of a material to be analyzed by observing and measuring the interaction of nuclear spins when placed in a powerful magnetic field. NMR
NMR spectroscopy is a technique used by chemists and biochemists to find the properties of organic molecules, although it is applicable to any kind of sample that contains nuclei possessing spin (Like 1H, 13C, 31P etc). The NMR can quantitatively analyze mixtures containing known compounds.
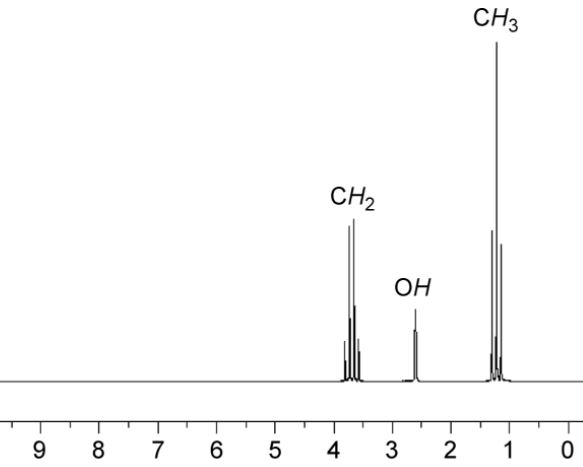
NMR spectra Information
- Chemical shift: Information about the composition of atomic groups within the molecule.
- Spin-Spin coupling constant: Information about adjacent atoms.
- Relaxation time: Information on molecular dynamics.
- Signal intensity: Quantitative information, e.g. atomic ratios within a molecule that can be helpful in determining the molecular structure, and proportions of different compounds in a mixture.
Components of NMR Spectroscopy
- Test tube holder
- Permanent magnet
- Sweep generator
- Radiofrequency transmitter
- RF detector
- Recorder
- Readout system –